单抗测序、De Novo Sequencing of mAb、单克隆抗体的从头测序
产品名称: 单抗测序、De Novo Sequencing of mAb、单克隆抗体的从头测序
英文名称: (De Novo Sequencing of mAb)
产品编号:
产品价格: 120000
产品产地: null
品牌商标: null
更新时间: null
使用范围: null
- 联系人 :
- 地址 : 江苏省苏州市园区星湖街218号生物纳米科技园A3楼319室
- 邮编 : 215123
- 所在区域 : 江苏
- 电话 : QQ:267***3817 点击查看
- 传真 : 点击查看
- 邮箱 : zhang.fengdan@prottech.com.cn
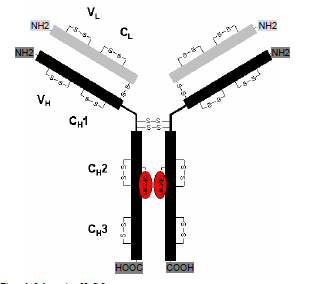
ProtTech®提供单抗全序列从头测序服务,我们建立了抗体从头测序的一系列方法并开发了十多个专有软件,已有完善的数据分析平台,我们能保证最终测序100%的准确。
1:单抗轻重链肽段覆盖率达到100%。
2:首次检测氨基酸准确率达到99.5%以上,活性如果有差异,我们免费复测,找出可能的差异点,保证客户在CHO细胞表达的抗体活性与原来相同。
3:适用于 人源、小鼠源、大鼠源、兔源等的IgG和IgM。
4:样品要求:蛋白纯度95%以上,蛋白量500ug 以上,周期4周。
ProtTech®offer the service for the complete de novo sequencing of mAb and other proteins. The service is based on the proprietary protein sequencing technology that we developed over many years.
We guarantee a result with a 100% sequencing coverage of the entire mAb molecules, a better than 99.5% accuracy and distinct assignment of Leu/Ile residues.
For de novo sequencing, the key issue is not mass spectrometer ( many different types of MS can do the job) but the bioinformatics software. We have 10+ proprietary software toolsused in our sequencing process.
Procedure:
The complete mAb de novo sequencing is often a very complex process, involving more than ten different proteolytic digestions, several steps of chemical derivatizations, NanoLC-ESI-MS/MS data acquisition, bioinformatics analysis with more than a dozen proprietary softwares, the manual spectra assignment, etc. In general the process contains the following steps:
1). Proteolytic digestion and chemical derivatization.
2). LCMS data acquisition.
3). Database search and peptide de novo sequencing.
4). Draft sequencing assembling.
5). Sequence gap filling.
6). Sequence error screening.
7). I/L determination.
8). Sequence validation.
Our mAb de novo sequencing technology can also apply to other proteins. However, changes in the process is often needed based on the nature of the protein.
Sample requirement:
Since more than ten different proteolytic reactions are carried out in the sequencing process, we prefer to have > 1mg mAb protein for each project, although ~500ug mAb sample is acceptable. A better than 90% purity is required.
The main concern about the quality of a sample is immunoglobin contamination since there is no easy way to separate contaminating antibodies from the mAb of interest, and there is not easy to determine if a peptide from contaminating peptides or from the mAb of interest. The major source of such a contamination is the serum in cell culture media.
Turn-Around Time:
It often takes five week to sequence one mAb sample.
